A. M. Melemed, D. A. Skiba, B. M. Gallant
Journal of Physical Chemistry C
2022 126 (2), 892-902
[publisher link]
Abstract
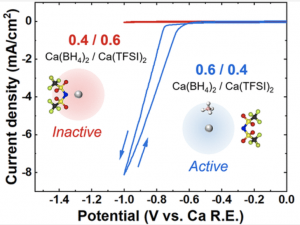 Learning how to tailor Ca2+ speciation and electroactivity is of central importance to engineer next-generation battery electrolytes. Using an exemplar dual-salt electrolyte, Ca(BH4)2 + Ca(TFSI)2 in THF, this work examines how to modulate a critical parameter proposed to govern electroactivity, the BH4–/Ca2+ ratio. The introduction of a more-dissociating source of Ca2+ via Ca(TFSI)2 drives respeciation of strongly ion-paired Ca(BH4)2, confirmed by ionic conductivity, Raman spectroscopy, and reaction microcalorimetry measurements, generating larger populations of charged species and enhancing plating currents. Ca plating is possible when [Ca(TFSI)2] < [Ca(BH4)2], and thus, BH4–/Ca2+ > 1, but a dramatic shutdown of plating activity occurs when [Ca(TFSI)2] > [Ca(BH4)2] (BH4–/Ca2+ < 1), directly evidencing the significance of coordination-shell chemistry on plating activity. Ca(BH4)2 + TBABH4 in THF, which enables enrichment of BH4– concentrations compared to Ca2+, is also examined; ionic conductivity and plating currents also increase compared to Ca(BH4)2/THF, with the latter related in part to a decrease in solution resistance. These findings delineate future directions to modulate Ca2+ coordination toward achieving both high plating activity and reversibility. Learning how to tailor Ca2+ speciation and electroactivity is of central importance to engineer next-generation battery electrolytes. Using an exemplar dual-salt electrolyte, Ca(BH4)2 + Ca(TFSI)2 in THF, this work examines how to modulate a critical parameter proposed to govern electroactivity, the BH4–/Ca2+ ratio. The introduction of a more-dissociating source of Ca2+ via Ca(TFSI)2 drives respeciation of strongly ion-paired Ca(BH4)2, confirmed by ionic conductivity, Raman spectroscopy, and reaction microcalorimetry measurements, generating larger populations of charged species and enhancing plating currents. Ca plating is possible when [Ca(TFSI)2] < [Ca(BH4)2], and thus, BH4–/Ca2+ > 1, but a dramatic shutdown of plating activity occurs when [Ca(TFSI)2] > [Ca(BH4)2] (BH4–/Ca2+ < 1), directly evidencing the significance of coordination-shell chemistry on plating activity. Ca(BH4)2 + TBABH4 in THF, which enables enrichment of BH4– concentrations compared to Ca2+, is also examined; ionic conductivity and plating currents also increase compared to Ca(BH4)2/THF, with the latter related in part to a decrease in solution resistance. These findings delineate future directions to modulate Ca2+ coordination toward achieving both high plating activity and reversibility.
|