Research
Solid Electrolyte Interphase (SEI) of Lithium Metal Anodes
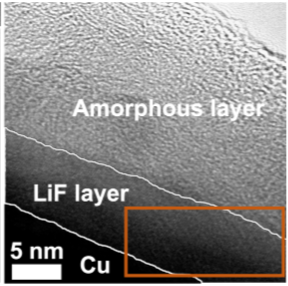 It is essential to move beyond today’s graphite anodes (372 mAh/g) to markedly increase the gravimetric energy of today’s Li-ion batteries. Switching to metallic lithium (Li, 3,861 mAh/g) has been a long-sought goal in the battery field, but Li anodes do not cycle with sufficiently high charge (Coulombic) efficiency (CE) at present, leading to CE values that fall short of targets (>99.9% over hundreds of cycles). Inefficiencies arise from the tendency of plated Li metal to react with nearly all battery electrolytes to form a derived interface, termed the Solid Electrolyte Interphase (SEI), which is typically < 50 nm, Li+ conductive and blocking to electrons. The SEI is fragile, reactive, and cannot perfectly buffer mechanical strains during Li plating and stripping, necessitating its continuous repair over cycling, which consumes electrolyte and Li inventory from the cell. This degree of repair, and correspondingly CE, are highly electrolyte dependent.
It is essential to move beyond today’s graphite anodes (372 mAh/g) to markedly increase the gravimetric energy of today’s Li-ion batteries. Switching to metallic lithium (Li, 3,861 mAh/g) has been a long-sought goal in the battery field, but Li anodes do not cycle with sufficiently high charge (Coulombic) efficiency (CE) at present, leading to CE values that fall short of targets (>99.9% over hundreds of cycles). Inefficiencies arise from the tendency of plated Li metal to react with nearly all battery electrolytes to form a derived interface, termed the Solid Electrolyte Interphase (SEI), which is typically < 50 nm, Li+ conductive and blocking to electrons. The SEI is fragile, reactive, and cannot perfectly buffer mechanical strains during Li plating and stripping, necessitating its continuous repair over cycling, which consumes electrolyte and Li inventory from the cell. This degree of repair, and correspondingly CE, are highly electrolyte dependent.
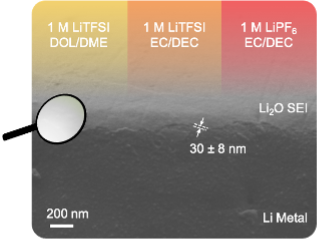
 Being electrolyte-derived, the SEI holds potential for indirect and direct engineering by selection or design of better electrolytes, additives, cycling protocols, and other stabilization methodologies. However, in spite of much research and correlative findings from the field on how to improve electrolytes, there is still a lack of universal design principles to rationally guide development of an improved SEI from the perspective of the solid-state chemistry, physics, and performance of the SEI itself.
Being electrolyte-derived, the SEI holds potential for indirect and direct engineering by selection or design of better electrolytes, additives, cycling protocols, and other stabilization methodologies. However, in spite of much research and correlative findings from the field on how to improve electrolytes, there is still a lack of universal design principles to rationally guide development of an improved SEI from the perspective of the solid-state chemistry, physics, and performance of the SEI itself.
 One of our approaches has been to develop a series of model SEI consisting of specific and common SEI phases, which allows for their properties to be directly measured with higher chemical specificity compared to a more-complex native interface. We have examined the transport, chemical reactivity, and electrochemical performance of such model interfaces. This platform is enabling parametrization of the SEI and comparison of SEI phases which inform a global perspective on which phases are more or less desirable for ion exchange, stability, and ultimately CE.
One of our approaches has been to develop a series of model SEI consisting of specific and common SEI phases, which allows for their properties to be directly measured with higher chemical specificity compared to a more-complex native interface. We have examined the transport, chemical reactivity, and electrochemical performance of such model interfaces. This platform is enabling parametrization of the SEI and comparison of SEI phases which inform a global perspective on which phases are more or less desirable for ion exchange, stability, and ultimately CE.
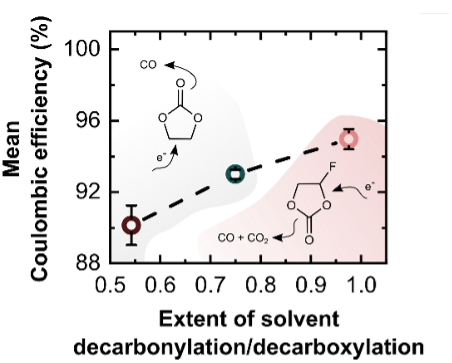
Other areas of research are looking at the chemical dynamics of SEI formation and repair. We employ operando gas chromatography and spectroscopy tools to examine SEI-building pathways and methodologies to tailor composition through coordination-shell engineering. These insights are being used to rationalize electrolyte selection and propose new additives that can contribute to improvements in CE.
Calcium Metal Anodes
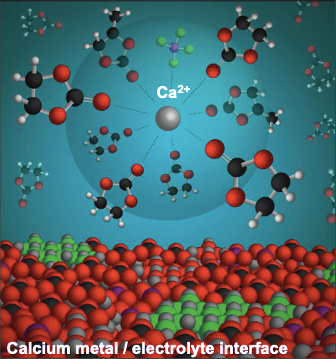 Given concerns about the low earth abundance of Li and graphite, there is growing interest in developing a beyond-Li materials basis for rechargeable batteries. Divalent batteries based on calcium (Ca) have received attention due to the element’s ∼2000-fold higher concentration in the Earth’s crust compared to Li, as well as attractive theoretical metrics if Ca metal is used as the anode. Ca metal has a low reduction potential (−2.87 V vs SHE), high gravimetric and volumetric capacities (1337 mAh/g and 2073 mAh/cc, respectively), and the possibility of safer operation given reports of dendrite-free deposition in certain electrolyte formulations. However, Ca anodes cannot be used arbitrarily in the wide range of electrolytes known to the battery community given high degrees of ion pairing and an enhanced tendency to form an SEI that is blocking to divalent Ca2+, a limitation that historically has slowed progress in Ca battery research.
Given concerns about the low earth abundance of Li and graphite, there is growing interest in developing a beyond-Li materials basis for rechargeable batteries. Divalent batteries based on calcium (Ca) have received attention due to the element’s ∼2000-fold higher concentration in the Earth’s crust compared to Li, as well as attractive theoretical metrics if Ca metal is used as the anode. Ca metal has a low reduction potential (−2.87 V vs SHE), high gravimetric and volumetric capacities (1337 mAh/g and 2073 mAh/cc, respectively), and the possibility of safer operation given reports of dendrite-free deposition in certain electrolyte formulations. However, Ca anodes cannot be used arbitrarily in the wide range of electrolytes known to the battery community given high degrees of ion pairing and an enhanced tendency to form an SEI that is blocking to divalent Ca2+, a limitation that historically has slowed progress in Ca battery research.
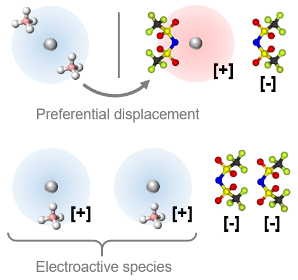 Recent years have seen growing interest in Ca electrochemistry spurred by breakthroughs in electrolyte engineering that have allowed reversible Ca plating/stripping to be demonstrated in a small number (<10) electrolytes with moderate Coulombic efficiency (~80 to 95%). There is opportunity for both fundamental studies and engineering improvements into all aspects of the metallic Ca anode: (1) the formation, composition, and structure of Ca SEI; (2) interactions of solvent molecules and anions in the Ca2+ solvation environment; and (3) the complex interplay of these factors in governing Ca2+ transport and deposition. Our group has performed research that examines the role of various interfaces (pre-existing, native, and synthetic) and cell configuration on Ca deposition behavior. We have also developed Ca2+ re-speciation techniques that can modify the distribution of solvation environments, meaningfully impacting Ca SEI and electrochemistry.
Recent years have seen growing interest in Ca electrochemistry spurred by breakthroughs in electrolyte engineering that have allowed reversible Ca plating/stripping to be demonstrated in a small number (<10) electrolytes with moderate Coulombic efficiency (~80 to 95%). There is opportunity for both fundamental studies and engineering improvements into all aspects of the metallic Ca anode: (1) the formation, composition, and structure of Ca SEI; (2) interactions of solvent molecules and anions in the Ca2+ solvation environment; and (3) the complex interplay of these factors in governing Ca2+ transport and deposition. Our group has performed research that examines the role of various interfaces (pre-existing, native, and synthetic) and cell configuration on Ca deposition behavior. We have also developed Ca2+ re-speciation techniques that can modify the distribution of solvation environments, meaningfully impacting Ca SEI and electrochemistry.
Fluorinated Conversion Reactions
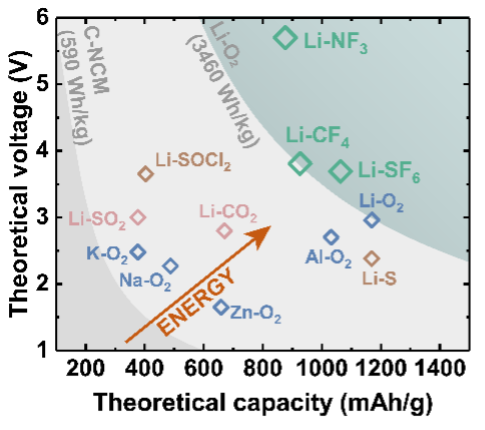 Exceeding the energy limits of today’s batteries will require fundamental innovation at the chemistry level to identify new types of reactions. We are working to expand the portfolio of conversion-type reactions with potential to underpin record-high energy density Li primary batteries (> 800 Wh/kg at a cell materials level) for diverse applications demanding energy, reliability and safety such as remote monitoring, undersea and space exploration, implantable medical devices and robotics. An example is the metal−gas battery based on Li−sulfur hexafluoride (SF6) conversion developed in our group, which demonstrated the feasibility of accessing the full oxidation state change of sulfur in an electrochemical reaction via 8-electron transfer reduction, yielding extensive lithium fluoride formation.
Exceeding the energy limits of today’s batteries will require fundamental innovation at the chemistry level to identify new types of reactions. We are working to expand the portfolio of conversion-type reactions with potential to underpin record-high energy density Li primary batteries (> 800 Wh/kg at a cell materials level) for diverse applications demanding energy, reliability and safety such as remote monitoring, undersea and space exploration, implantable medical devices and robotics. An example is the metal−gas battery based on Li−sulfur hexafluoride (SF6) conversion developed in our group, which demonstrated the feasibility of accessing the full oxidation state change of sulfur in an electrochemical reaction via 8-electron transfer reduction, yielding extensive lithium fluoride formation.
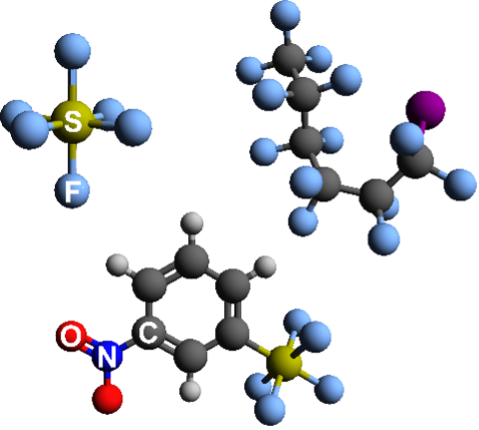 Recent work has developed advanced liquid fluorinated reactants that achieve in excess of 8 e−/molecule and up to 15 e−/molecule at high reactant concentrations as catholytes, and can be hybridized with existing solid conversion materials to substantially boost energy density of commercial batteries by an estimated 20%. By examining discharge products and pathways, our studies have examined the science of electrochemical fluoride conversion in non-transition metal-based systems, including fluoride bond activation in S−F, N−F, and C−F bonding environments, and learned how to harness and design these potent reactions to yield new classes of energy storage systems. Ongoing efforts are examining how to exploit these reactions for rechargeability.
Recent work has developed advanced liquid fluorinated reactants that achieve in excess of 8 e−/molecule and up to 15 e−/molecule at high reactant concentrations as catholytes, and can be hybridized with existing solid conversion materials to substantially boost energy density of commercial batteries by an estimated 20%. By examining discharge products and pathways, our studies have examined the science of electrochemical fluoride conversion in non-transition metal-based systems, including fluoride bond activation in S−F, N−F, and C−F bonding environments, and learned how to harness and design these potent reactions to yield new classes of energy storage systems. Ongoing efforts are examining how to exploit these reactions for rechargeability.
Electrochemical CO2 Management
 To limit global temperature rise to below 2 °C, the IEA’s Sustainable Development Scenario indicates that installed CO2 capture systems must scale globally from approximately 40 megatons in 2020 to 10 gigatons/year in 2070. The benchmark CO2 capture process, which operates via retrofit to point-source emitters, relies on solutions of aqueous amines that capture CO2 around 40 °C and employs heating to regenerate the CO2-loaded sorbent at around 120 °C. The technology has been extensively investigated for almost 100 years and has reached technical maturity, but remains highly energy and capitally intensive.
To limit global temperature rise to below 2 °C, the IEA’s Sustainable Development Scenario indicates that installed CO2 capture systems must scale globally from approximately 40 megatons in 2020 to 10 gigatons/year in 2070. The benchmark CO2 capture process, which operates via retrofit to point-source emitters, relies on solutions of aqueous amines that capture CO2 around 40 °C and employs heating to regenerate the CO2-loaded sorbent at around 120 °C. The technology has been extensively investigated for almost 100 years and has reached technical maturity, but remains highly energy and capitally intensive.
On the other hand, electrochemical CO2 conversion, powered by renewable energy, has potential to provide carbon-neutral chemicals and fuels and displace current fossil-based sources, but is also energy intensive. These challenges are compounded by the fact that capture and conversion have historically been developed as standalone, decoupled processes, such that inefficiencies and energy requirements rapidly accrue.
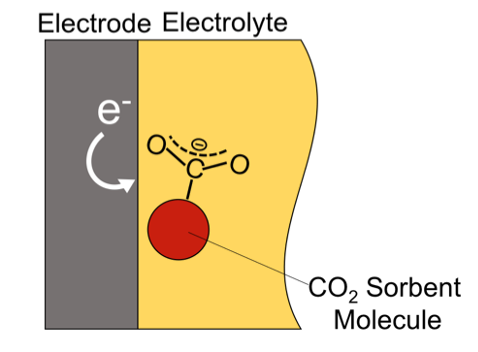
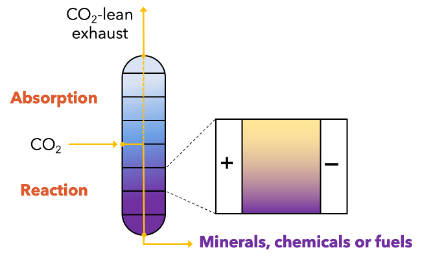 Our group is developing electrochemical processes that integrate capture-conversion to yield a range of products, from mineral solids (carbonates) to chemicals (e.g., carbon monoxide) and fuels. We demonstrated the feasibility of conducting electrochemical reduction of amine-CO2 adducts in a capture solution to drive CO2-derived products. There is great need and opportunity to expand the basic scientific understanding of amine-based capture in electrochemical environments, including elucidating reactant speciation, reactant and intermediate transport, interfacial processes and factors that govern product branching and selectivity. Additional efforts are investigating electrochemical separations with application to both point-source emitters and direct capture of CO2 from air.
Our group is developing electrochemical processes that integrate capture-conversion to yield a range of products, from mineral solids (carbonates) to chemicals (e.g., carbon monoxide) and fuels. We demonstrated the feasibility of conducting electrochemical reduction of amine-CO2 adducts in a capture solution to drive CO2-derived products. There is great need and opportunity to expand the basic scientific understanding of amine-based capture in electrochemical environments, including elucidating reactant speciation, reactant and intermediate transport, interfacial processes and factors that govern product branching and selectivity. Additional efforts are investigating electrochemical separations with application to both point-source emitters and direct capture of CO2 from air.
