H. Gao, A. Sevilla, B. M. Gallant
Journal of the Electrochemical Society, 2022
★ Editor’s Choice
[publisher link]
Abstract
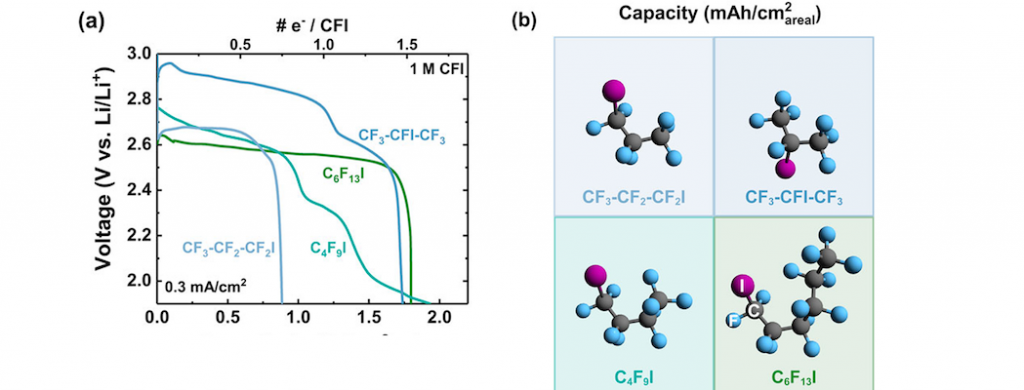
While Li−carbon monofluoride (CFx) is the current energy leader among primary batteries, the technology is maturing, motivating further fundamental study of Li battery chemistry based on C−F redox. This study examines the possibility to conduct multi-electron carbon reduction using a candidate class of liquid CFx analogues, perfluoroalkyl iodides (CnF2n+1I, with F/C ratios of x > 2), in supporting electrolyte as catholytes for Li cells. The large, polarizable iodine supports electrochemical reduction with concerted F− ligand expulsion, forming lithium fluoride (LiF) as the main solid discharge product. Under initial conditions (1 M reactant and 0.3 mA cm−2 in dimethylsulfoxide), only limited defluorination (1.5 e−/molecule) is accessed. Governing factors for C−F bond redox are further investigated, including reactant concentration, discharge rate, temperature, and solvent properties (e.g. catholyte viscosity). A maximum of 8 e−/C6F13I, or 8/13 available F, is accessible in the voltage range 2.8−1.9 V vs Li/Li+ with low reactant concentrations (0.1 M) and rates (20 μA cm−2). The data indicate that multiple handles exist to tailor extended C−F bond activation in these reactants. However, premature reaction termination caused by deactivation of intermediates, which is particularly exacerbated at higher concentrations and/or rates, is likely to be a persistent challenge for practical applications.