K.-H. Kim, G. M. Hobold, K. J. Steinberg, and B. M. Gallant
Confinement Effects of Hollow Structured Pt–Rh Electrocatalysts toward Complete Ethanol Electrooxidation
ACS Nano, 2023, DOI: 10.1021/acsnano.3c05334
[publisher link]
Abstract
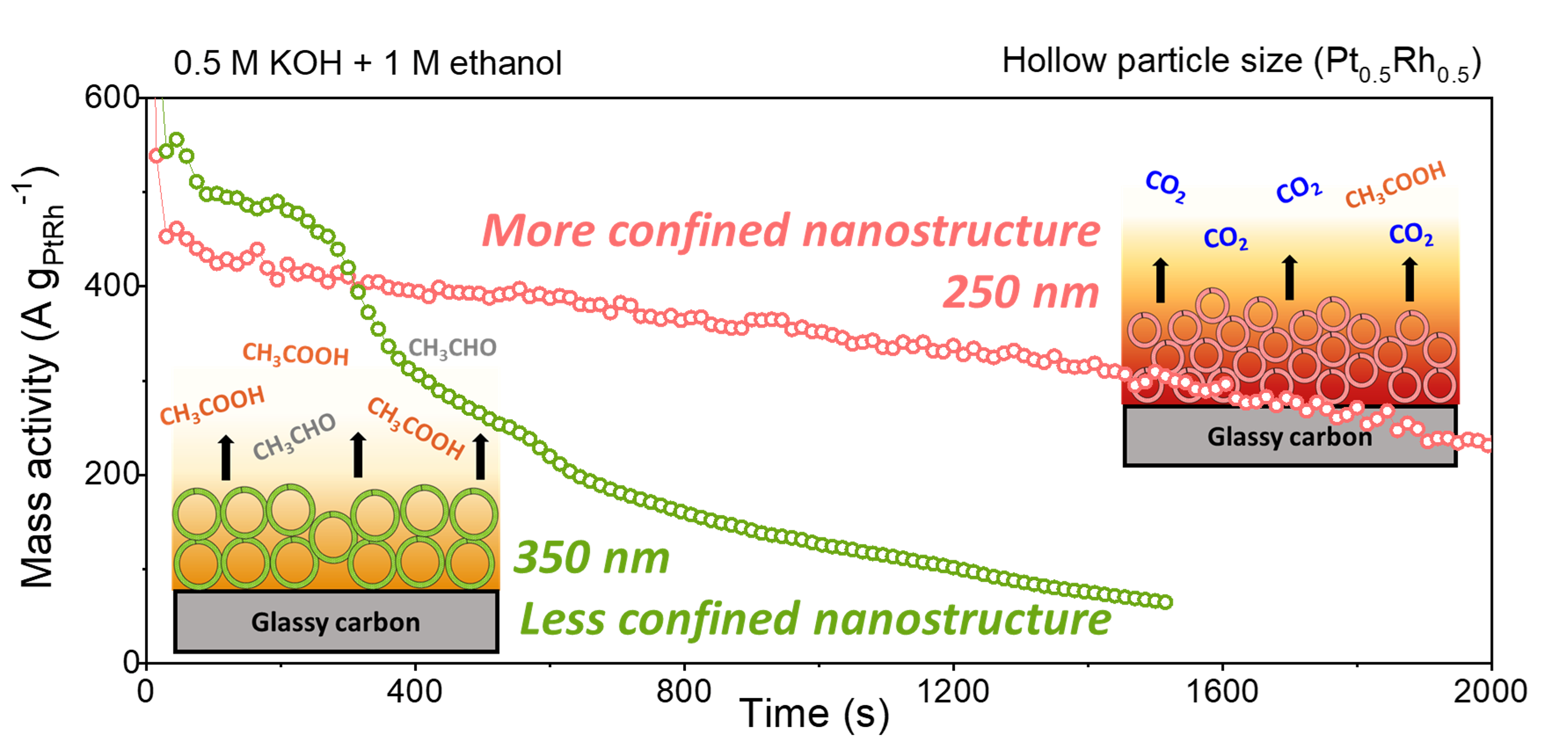
In the anodic ethanol oxidation reaction (EOR) for direct ethanol fuel cells, the coverage of hydroxide (OHads) is a major adsorbent competing with C–C bond cleavage, which is necessary for complete ethanol oxidation (C1-pathway) and durability. Beyond utilizing a less-alkaline electrolyte that causes ohmic losses, an alternative strategy to optimize OHads coverage is to intentionally exploit local pH changes near the electrocatalyst surface that are governed by a combination of released H+ during EOR and OH– mass transport from the bulk solution. Here, we manipulate the local pH swing by fine-tuning the electrode porosity with Pt1–xRhx hollow sphere electrocatalysts based on particle size (250 and 350 nm) and mass loading. With the smaller size of 250 nm, Pt0.5Rh0.5 (∼50 μg cm–2) shows a high activity of 1629 A gPtRh–1 (2488 A gPt–1) in a 0.5 M KOH-containing electrolyte, which is ∼50% higher than the most active binary catalysts to date. Moreover, a higher C1-pathway Faradaic efficiency (FE) of 38.3% and 80% longer durability are achieved with a 2-fold increase in mass loading. In the more porous electrodes, a local acidic environment created by hindered OH– mass transport better optimizes OHads coverage, providing more active sites for the desired C1-pathway and a continuous EOR.

Recent Comments