H. Gao, K. Yoshinaga, K. J. Steinberg, T. M. Swager, and B. M. Gallant
Cascade Defluorination of Perfluoroalkylated Catholytes Unlocks High Lithium Primary Battery Capacities
Advanced Energy Materials, 2023, DOI: 10.1002/aenm.202301751
[publisher link]
Abstract
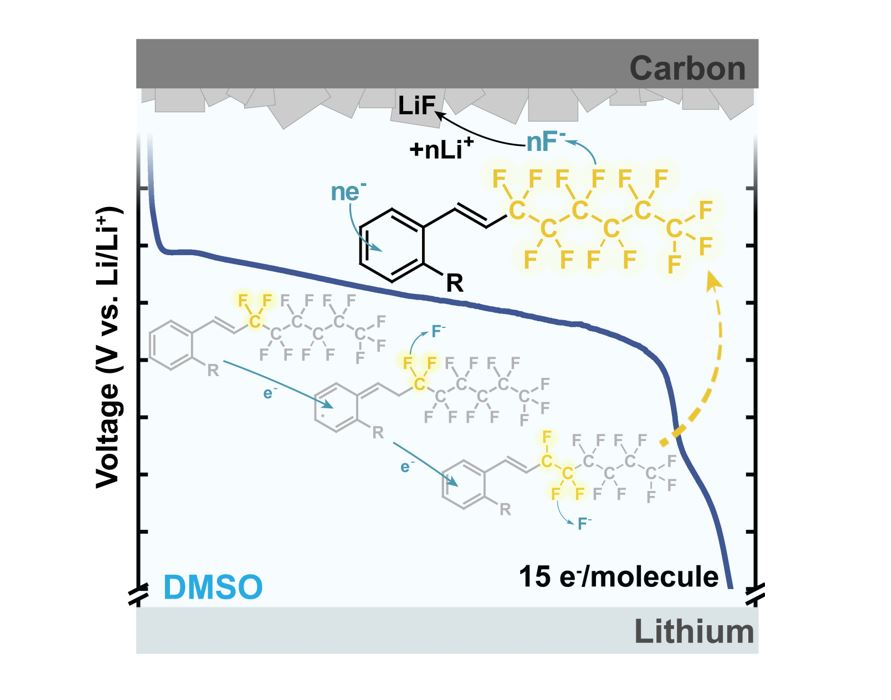
Exceeding the energy density of lithium−carbon monofluoride (Li−CFx), today’s leading Li primary battery, requires an increase in fluorine content (x) that determines the theoretical capacity available from C−F bond reduction. However, high F-content carbon materials face challenges such as poor electronic conductivity, low reduction potentials (<1.3 V versus Li/Li+), and/or low C−F bond utilization. This study investigates molecular structural design principles for a new class of high F-content fluoroalkyl-aromatic catholytes that address these challenges. A polarizable conjugated system—an aromatic ring with an alkene linker—functions as electron acceptor and redox initiator, enabling a cascade defluorination of an adjacent perfluoroalkyl chain (RF = −CnF2n+1). The synthesized molecules successfully overcome premature deactivation observed in previously studied catholytes and achieve close-to-full defluorination (up to 15/17 available F), yielding high gravimetric capacities of 748 mAh g−1fluoroalkyl-aromatic and energies of 1785 Wh kg−1fluoroalkyl-aromatic. The voltage compatibility between fluoroalkyl-aromatics and CFx enables design of hybrid cells containing C−F redox activity in both solid and liquid phases, with a projected enhancement of Li–CFx gravimetric energy by 35% based on weight of electrodes+electrolyte. With further improvement of cathode architecture, these “liquid CFx” analogues are strong candidates for exceeding the energy limitations of today’s primary chemistries.

Recent Comments