A. M. Melemed, D. A. Skiba, K. J. Steinberg, K.-H. Kim, and B. M. Gallant
Impact of Differential Ca2+ Coordination in Borohydride-Based Electrolyte Blends on Calcium Electrochemistry and SEI Formation
DOI: 10.1021/acs.jpcc.3c03800
[publisher link]
Abstract
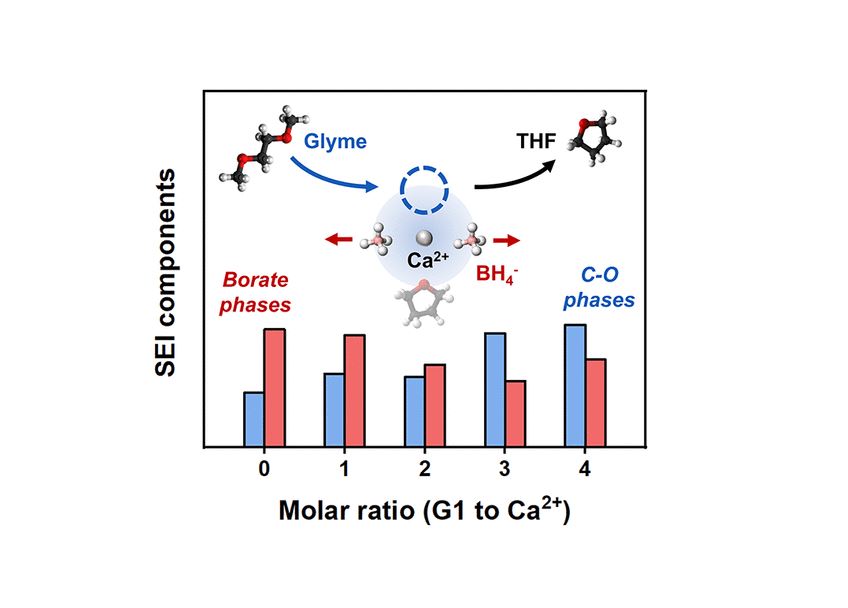
Despite growing interest in calcium (Ca)-based batteries for sustainable energy storage, there are a limited number of electrolytes that enable the reversible plating/stripping of Ca metal. Consequently, an understanding of the interplay between electrolyte formulation, solid electrolyte interphase (SEI) composition, and electrochemical performance lags behind that of other alkali and alkaline-earth batteries. In this context, this study examines how plating/stripping behavior and SEI formation are modulated by differential changes in solvent composition and the resulting Ca2+–anion speciation in borohydride electrolytes comprising ether solvent blends. Starting with the baseline electrolyte 1 M Ca(BH4)2 in tetrahydrofuran (THF), THF was systematically replaced by 1,2-dimethoxyethane (G1) or bis(2-methoxyethyl) ether (G2) over a range of Gx:Ca2+ molar ratios (0–4:1 G1:Ca2+ or 0–3:1 G2:Ca2+). Replacement of THF by glymes increased the plating overpotential and decreased the Coulombic efficiency of Ca plating/stripping, and a marked decrease in electrochemical activity occurred at a composition threshold for each glyme. Comparison between the X-ray photoelectron spectra of electrolyte-soaked Ca and electrodeposited Ca revealed a relative increase in organic C–O species and a decrease in borate species in the electrochemically-formed SEI with increased glyme proportion in the electrolyte. NMR and Raman spectroscopy connected SEI composition to differential Ca2+ coordination, as glymes displace THF from the Ca2+ coordination environment, weaken Ca2+–BH4– interactions, and prompt BH4– reorganization. Overall, BH4–-facilitated solvent decomposition governs Ca electrochemistry in these systems, as coordinated THF promotes beneficial borate formation but coordinated glymes limit such phases, leading to Ca2+-blocking phases in the SEI. These findings delineate future directions to modulate Ca(BH4)2 coordination toward improving electrolyte reversibility by considering the O-donating ability of the coordinating solvent.

Recent Comments