G. Leverick, E. M. Bernhardt, A. I. Ismail, J. H. Law, A. Arifutzzaman, M. K. Aroua, and B. M. Gallant
ACS Catalysis 2023, 13, 12322–12337. Doi: 10.1021/acscatal.3c02500
★ Featured in MIT News
[publisher link]
Abstract
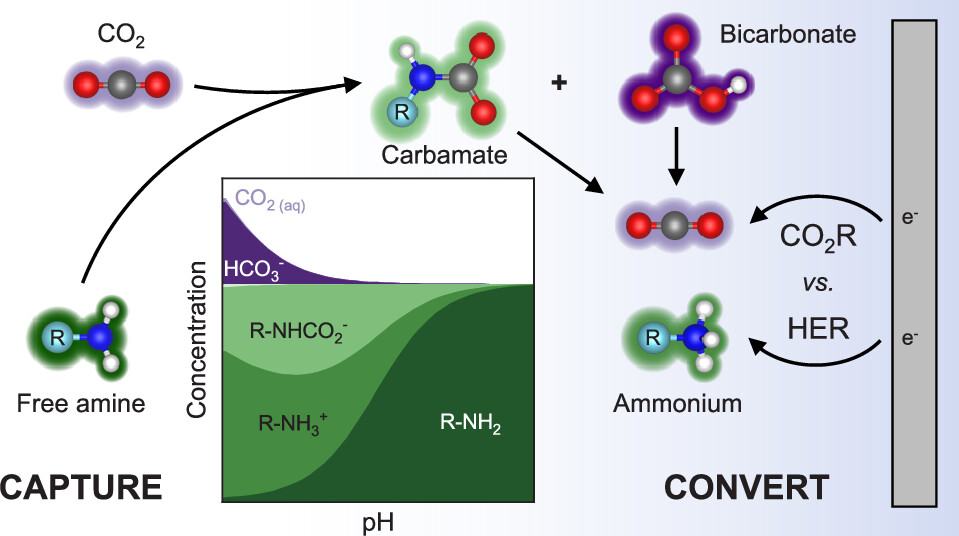
Electrochemical reactive capture of CO2, which integrates CO2 capture with its conversion directly from amine and other capture solutions, is of growing interest to enable net zero and eventually negative greenhouse gas emissions. While integration has been proposed to mitigate certain energy penalties and inefficiencies that accrue when capture and conversion are decoupled, integration introduces considerable complexity to the electrochemical process due to the number of possible reactant participants, especially in an aqueous-based capture solution. Moreover, the influence of amine-based sorbents on CO2 reduction (CO2R) mechanisms is not well-understood, making rational design elusive at present. In this work, we reveal the governing parameters and active species in amine-mediated CO2 conversion as an essential initial step toward improving these processes. We first demonstrate the critical influence of CO2 partial pressure of the capture stream on the resulting solution pH, which directly affects amine speciation and the Faradaic efficiency of CO production on Ag. Moreover, by considering amines of different pKa and with different propensities to form the amine-CO2 adduct carbamate, we show that dissolved CO2 is the active species for CO2R in amine-containing solutions, enabling some capture solutions to have comparable CO2R selectivity and kinetics to amine-free bicarbonate solutions. As a result, amines can serve as a reservoir of dissolved inorganic carbon that can replenish dissolved CO2 as it is consumed during CO2R, alleviating mass transfer/transport limitations without directly participating electrochemically.

Recent Comments