F.-Y. Kuo, S. E. Jerng, and B. M. Gallant
Dual Salt Cation-Swing Process for Electrochemical CO2 Separation
ACS Central Science 2023, DOI: 10.1021/acscentsci.3c00692
[publisher link]
Abstract
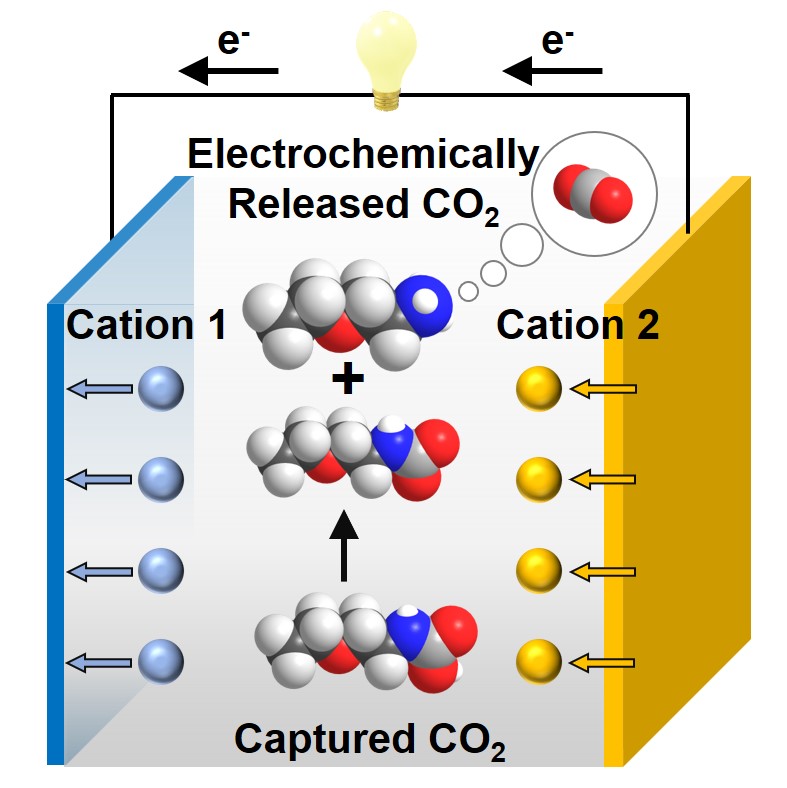
Electrochemical CO2 separations, which use electricity rather than thermal energy to reverse sorption of CO2 from concentrated point sources or air, are emerging as compelling alternatives to conventional approaches given their isothermal, ambient operating conditions, and ability to integrate with renewable energy inputs. Despite several electrochemical approaches proposed in previous studies, further explorations of new electrochemical CO2 separation methods are crucial to widen choices for different emissions sources. Herein, we report an electrochemical cation-swing process that is able to reversibly modulate the CO2 loading on liquid amine sorbents in dimethyl sulfoxide (DMSO) solvent. The process exploits a reversible carbamic acid-to-carbamate conversion reaction that is induced by changing the identity of Lewis acid cations (e.g. K+, Li+, Ca2+, Mg2+, and Zn2+) coordinated to the amine-CO2 adduct in the electrolyte. Using ethoxyethylamine (EEA) as a model amine, we present NMR-based speciation studies of carbamic acid-to-carbamate conversion as a function of amine/salt concentrations and cation identity. The reaction is further probed using gas-flow reaction microcalorimetry, revealing the energetic driving forces between cations and the amine-CO2 adduct that play a key role in the described re-speciation. A prototype electrochemical cell was further constructed comprising a Prussian white (PW) potassium (K+) intercalation cathode, zinc (Zn) foil anode, and EEA/DMSO electrolyte containing a dual KTFSI/Zn(TFSI)2 salt. A low CO2 separation energy of ∼22–39 kJ/mol CO2 (0.1–0.5 mA cm–2) was achieved with a practical CO2 loading delta of ∼0.15 mol CO2/mol amine. Further optimizations in electrolyte design and cell architectures toward continuous CO2 capture-release are expected to enhance rate performance while retaining favorable separation energies.

Recent Comments